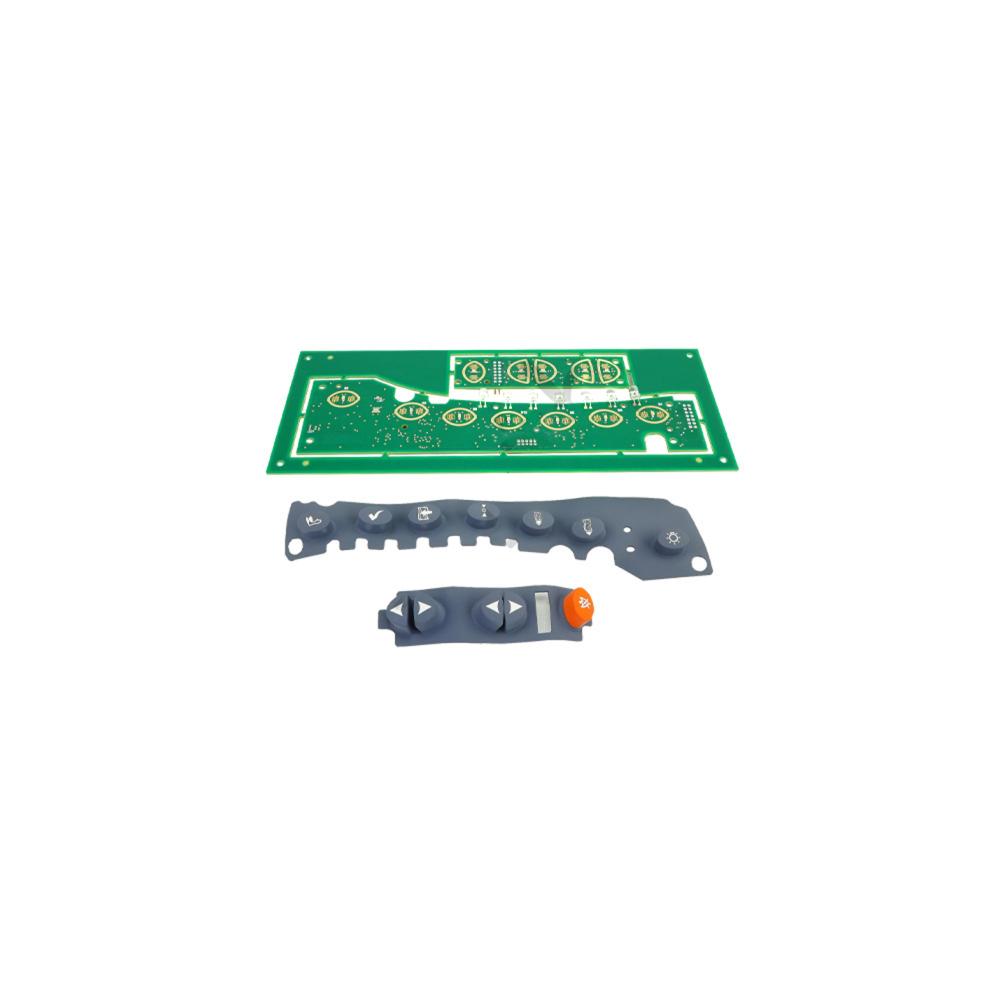
Corometrics 250 Keypad and Volume Pad with Elastomer - RoHS
| 2025177-073 | |
| 2025177-023 | |
| Cuidado materno-infantil | |
| Corometrics 250CX | |
| GE HealthCare Colombia S.A.S | |
| GE HealthCare | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Características
- High quality keypad and volume pad
- Equipped with main user interface Printed Circuit Board (PCB)
- Long life span
- Optimized maintenance kit
Descripción del producto
The Corometrics 250 Keypad and Volume Pad with Elastomer is a field service kit that is a specially designed and intended for use in Corometrics™ 250 series monitor and other medical equipment as applicable. The service kit is comprised of a main user interface Printed Wiring Assembly (PWA), user interface main keypad, side keypad, product identification label and packing label. The part can be used as a Field Replaceable Unit (FRU) during servicing and maintenance. The main keypad is engraved with names NIBP start/stop button, test button, mark (offset), Uterine Activity (UA) reference button, paper advance button, record button, power indicator, record indicator and light button. The volume/alarm keypad is engraved with names FHR2 volume decrease button, FHR2 volume increase button, FHR1 volume decrease button, Fetal Heart Rate (FHR1) volume increase button and alarm silence button. The main user interface board is the central processing unit for the monitor unit. The volume/alarm keypad board has an elastomeric property and has backlight LEDs for each keypad of the front panel. The user-interface keypad board interface with the main board is through an RS-232 interface. The keypad is made of high quality clear silicone rubber material that has high tensile strength, good tear resistance and excellent chemical resistant properties, which makes it well suited for modern medical applications and demanding applications. The service parts are securely packed inside a high quality corrugated box and are supported by bubble wrap and antistatic bags to avoid physical damage during transit. The part is RoHS compliant and is approved for today’s safety standards.
Características adicionales
- Easy to fix
- Durable
- Superior heat and compression resistance
- High tensile and tear resistance
- Chemical resistant
- Flame resistant
- Packed with carton box and bubble wrap
- Includes self adhesive product identification and shipping labels
- Environmental contaminant free materials used
- RoHS compliant
Artículo equivalente:
Tenemos las siguientes piezas equivalentes disponibles para usted. Estas son piezas de reemplazo compatibles. Estas piezas sin un hipervínculo se enumeran solo como referencia y no están disponibles para su compra en línea. Para obtener información adicional, comuníquese con el servicio al cliente
| Artículo equivalente | Detalles de parte |
|---|---|
| 2025177-023 | FRU, coro 250, teclado y almohadilla vol con elastómero |